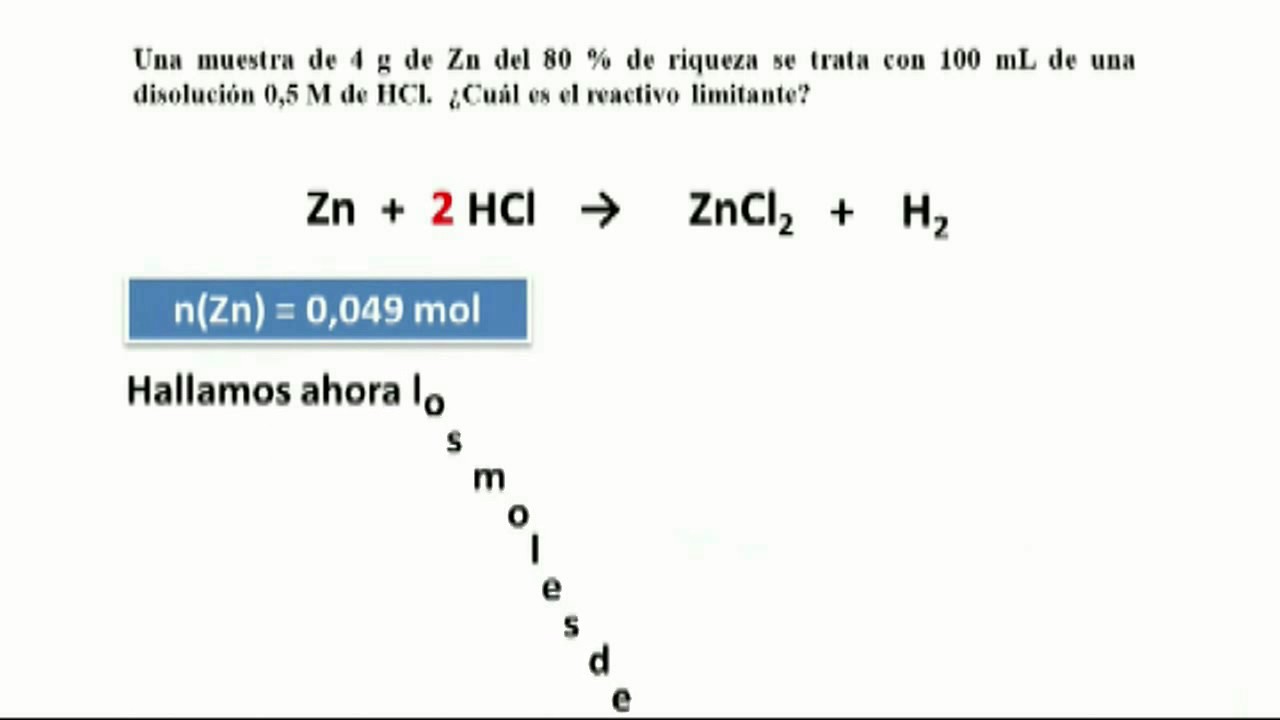
Zinc catalysed electrophilic C–H borylation of heteroarenes - Chemical Science (RSC Publishing) DOI:10.1039/D1SC01883C

Treatment of benzaldehyde (C_6H_5CHO) with Zn(Hg) in aqueous HCl forms a compound Z that has a molecular ion at 92 in its mass spectrum. Z shows absorptions at 3150-2950, 1605, and 1496

How to Balance H2SO4 + Zn = ZnSO4 + H2 (Sulfuric Acid + Zinc) | How to Balance H2SO4 + Zn = ZnSO4 + H2 (Sulfuric Acid + Zinc) Balancing equations could

Trimetallic Cu–Ni–Zn/H-ZSM-5 Catalyst for the One-Pot Conversion of Levulinic Acid to High-Yield 1,4-Pentanediol under Mild Conditions in an Aqueous Medium | ACS Catalysis
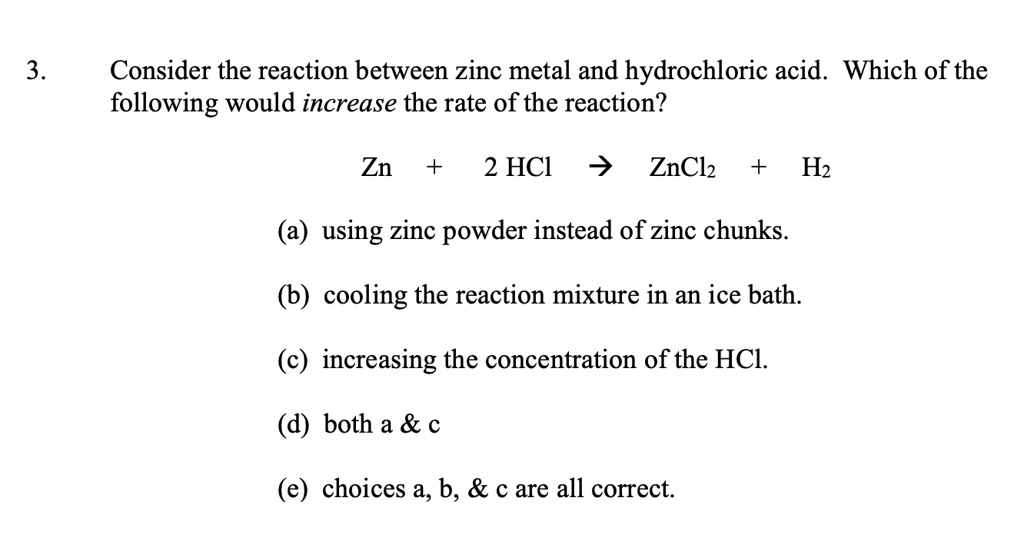
SOLVED: Consider the reaction between zinc metal and hydrochloric acid. Which of the following would increase the rate of the reaction? 3. ZnCl2 2. HCl Zn H2 using zinc powder instead of
Consider the following reactions (unbalanced) Zn + hot conc. H2SO4 →G + R + X - Sarthaks eConnect | Largest Online Education Community

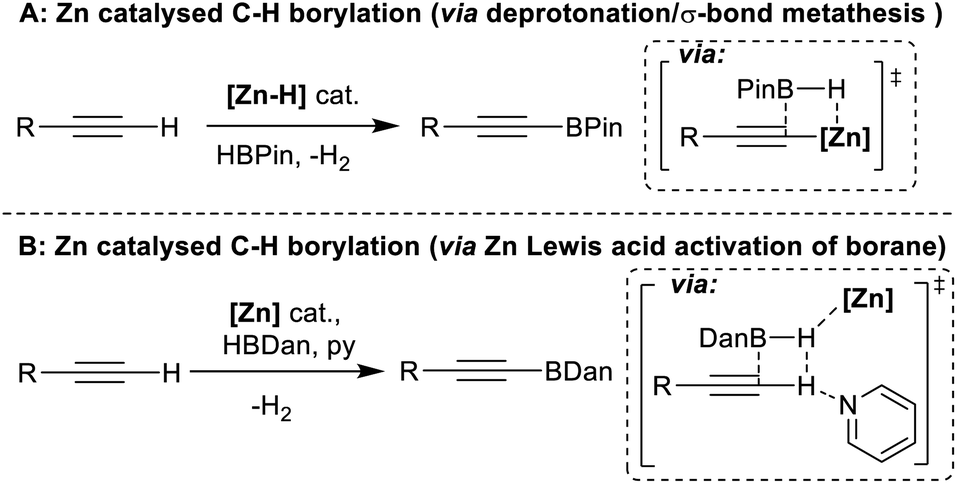
Hydrosilane σ‐Adduct Intermediates in an Adaptive Zinc‐Catalyzed Cross‐dehydrocoupling of Si−H and O−H Bonds - Patnaik - 2021 - Chemistry – A European Journal - Wiley Online Library

SOLVED: In the reaction Zn + H+ —> Zn2+ + H2, Zn is oxidized and H is reduced. How many electrons would be produced and used in the balanced half-reactions? Complete the
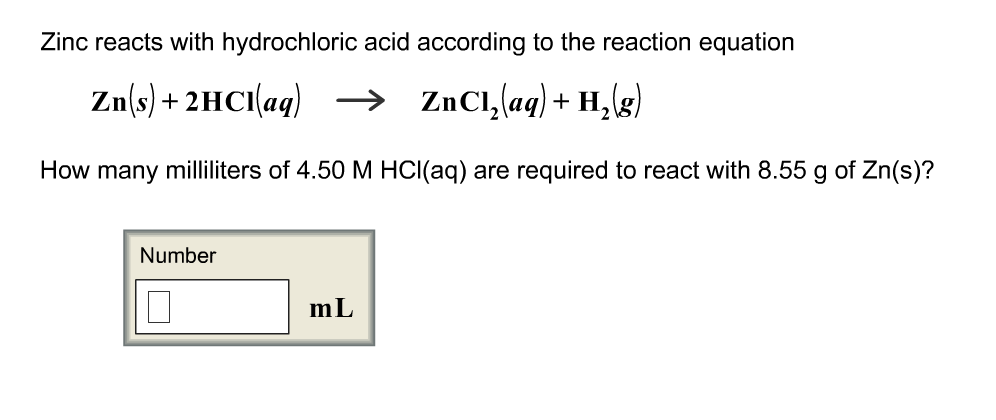
43. Balance the following equation: Zn + (H+) —> (Zn+2) + H2 (Zinc reacts with hydrogen ion to give Zinc ion and Hydrogen gas.)


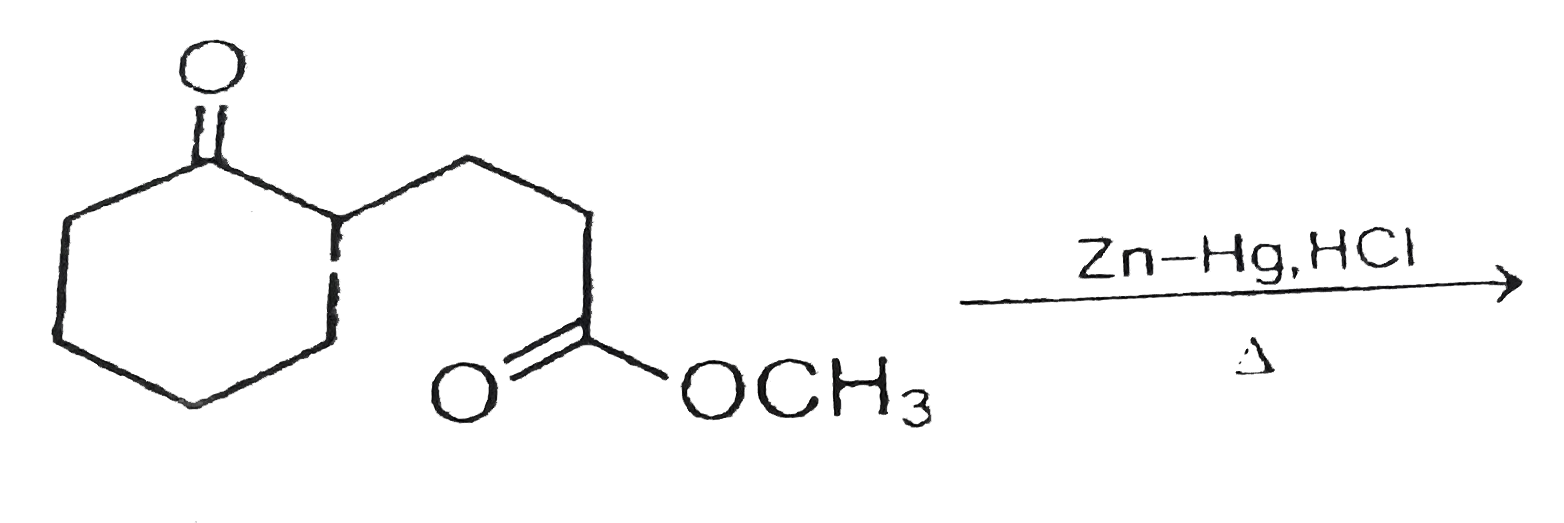



![In the reaction, C6H5COCH3 [Zn - Hg/conc. HCl][H]X . X is: In the reaction, C6H5COCH3 [Zn - Hg/conc. HCl][H]X . X is:](https://dwes9vv9u0550.cloudfront.net/images/7671796/5bdff3d8-c0c9-4fc8-89cd-f9235a0c1b30.jpg)